Learning Outcomes
By the end of this lesson, students will be able to:
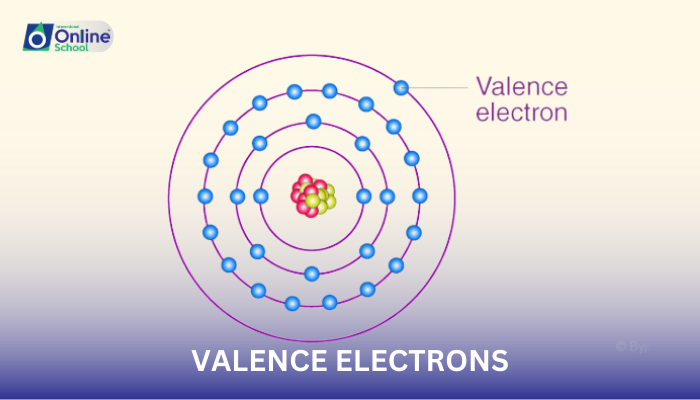
i. Define and explain the concept of valence electrons, the electrons involved in chemical bonding.
ii. Identify and determine the number of valence electrons in an atom using the periodic table.
iii. Recognize the relationship between the position of an element in the periodic table and its number of valence electrons.
iv. Understand how valence electrons influence the chemical reactivity of elements.
v. Apply the knowledge of valence electrons to predict and explain the bonding behavior of elements.
Introduction
The periodic table, a cornerstone of chemistry, serves as a comprehensive arrangement of elements, revealing a profound order and patterns that govern their properties and behavior. Among these, valence electrons stand out as key players in determining the chemical landscape.
i. Valence Electrons: The Gatekeepers of Chemical Bonds
Valence electrons, the outermost electrons in an atom's orbitals, play a pivotal role in chemical bonding, the process by which atoms unite to form molecules. These electrons are the primary participants in electron sharing, the mechanism that underlies covalent bonds, and electron transfer, the foundation of ionic bonds.
ii. Locating Valence Electrons in the Periodic Table
The periodic table provides a valuable tool for determining the number of valence electrons in an atom:
Group Number: For most elements, the number of valence electrons corresponds to the group number in the periodic table.
Exceptions: For transition metals (Groups 3-12), the valence electron configuration may differ from the group number, requiring additional consideration.
iii. Valence Electrons and Chemical Reactivity
Valence electrons significantly influence the chemical reactivity of elements:
Electron Sharing: Elements with similar valence electron configurations tend to share electrons readily, forming covalent bonds.
Electron Transfer: Elements with a high electronegativity, the ability to attract electrons, may gain electrons from elements with low electronegativity, forming ionic bonds.
Predicting Bonding Behavior: The number of valence electrons provides a starting point for predicting the type of bond an element can form.
Examples of Valence Electrons and Bonding
Sodium (Na): With one valence electron, sodium readily loses this electron to form ionic bonds with nonmetals, such as chlorine (Cl).
Oxygen (O): With six valence electrons, oxygen can share electrons to form covalent bonds with various elements, such as hydrogen (H) in water (H2O) and carbon (C) in carbon dioxide (CO2).
Neon (Ne): With a full valence shell of eight electrons, neon exhibits low reactivity and forms no stable compounds.
Valence electrons, the outermost electrons in an atom, hold immense significance in the realm of chemistry. By understanding their role in chemical bonding, their relationship to the periodic table, and their influence on chemical reactivity, we gain valuable insights into the behavior of elements and their ability to form diverse compounds, further enriching our understanding of the intricate tapestry of chemistry.